Have you seen the expression hcooch ch2 h2o and wondered, “What’s that supposed to mean?” You’re definitely not the only one. This kind of formula might look strange or confusing at first, but don’t worry—we’re going to break it down together. This guide makes it super simple by using easy examples, clear language, and real meaning behind the chemistry. Whether you’re studying science, helping your child with homework, or just curious, this article explains exactly what hcooch ch2 h2o stands for. It’s more than just random letters. It shows a small but important chemical reaction that has meaning in science and everyday life. We’ll explain everything—from the molecules involved to how this reaction helps teach organic chemistry. Let’s get started and learn all about hcooch ch2 h2o step by step.
What Does hcooch ch2 h2o Mean in Chemistry?
The formula hcooch ch2 h2o shows a chemical reaction between three different molecules. Each part has meaning. First, HCOOH is the formula for formic acid, which is a small and simple organic acid. It is found in ant stings and even some plants. Next, CH2 stands for a methylene group, which has one carbon atom and two hydrogen atoms. Lastly, H2O is good old-fashioned water. In this reaction, formic acid and the methylene group come together. There’s a chemical change, and water is one of the end results. Scientists use examples like this to teach how molecules join, split, and create something new. So, next time you hear someone mention hcooch ch2 h2o, you’ll know it’s showing a basic organic reaction involving formic acid, methylene, and water.
Breaking Down HCOOH: What Is Formic Acid?
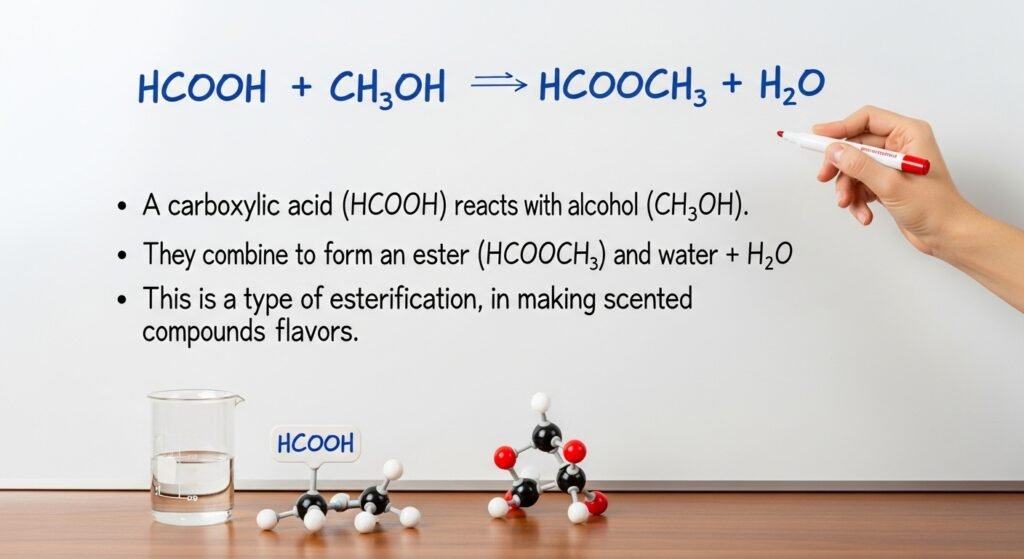
Let’s look closer at the first part of the hcooch ch2 h2o reaction: HCOOH. That’s the formula for formic acid, a very small molecule made of hydrogen, carbon, and oxygen. It’s the simplest kind of acid in a group known as carboxylic acids. Where is it found in the real world? Formic acid appears in ant venom and some stinging plants. If you’ve ever been bitten by an ant, that slight burn might have come from formic acid. It’s also used in farming and industry to preserve animal feed and tan leather. In chemistry, HCOOH is special because it reacts easily with other substances. It sets the stage for many acid-based reactions, like the one shown in hcooch ch2 h2o. Even though it’s just a five-letter formula, its power in chemistry and biology is impressive.
What Does CH₂ Mean in hcooch ch2 h2o?
Now, let’s look at CH2, the middle part of the formula hcooch ch2 h2o. CH2 is called a methylene group. It has one carbon atom and two hydrogen atoms. Methylene groups often act like tiny puzzle pieces in organic chemistry. They like to bond with other groups to form more complex molecules. CH2 is very reactive, which means it wants to connect with other chemicals. It doesn’t exist alone for long and is usually part of a larger molecule. In the reaction we’re looking at, CH2 likely comes from another compound or appears briefly during the change. Its job could be to help link or transform molecules like HCOOH into something new, with H2O as a result. Learning about CH2 helps us understand how molecules grow, split, and interact in living things, as well as in the lab.
The Final Product: Why H₂O Matters in This Equation
The last part of the expression hcooch ch2 h2o is H2O, the formula for water. Water is not just important to life—it’s also important in chemistry. During many organic reactions, molecules join together and release water as a by-product. This kind of result is often called a condensation reaction. In the case of hcooch ch2 h2o, H2O shows that something new has formed. Water comes from parts of the formic acid and methylene group bonding. It tells chemists that a change happened and helps to clue them in on what reaction type they’re observing. Simple as it looks, producing water during a reaction can have a big meaning. It shows that energy moved, atoms switched around, and a new compound likely appeared.
Is hcooch ch2 h2o a Real Reaction?
You may be wondering, “Is hcooch ch2 h2o actually a full reaction?” That’s a great question! In real chemistry, things are often more detailed. This expression is simplified. It gives a glimpse of how small molecules interact, but full reactions are more complex. In labs, chemists often add heat, pressure, or catalysts to help reactions happen. Also, the actual compounds may have extra atoms or groups to help balance the equation. So while hcooch ch2 h2o explains the basic idea behind a reaction, many small steps or extra parts may be involved. It’s not wrong—it’s just basic, like saying flour + water = dough. Good enough to get the idea, but missing a few in-between steps.
Why Chemists Use Reactions Like hcooch ch2 h2o
Even though the formula hcooch ch2 h2o is simple, it helps students and chemists learn some key concepts. It shows how acids like formic acid can react, how small groups like CH₂ behave, and how water can form as a result. Reactions like this are especially useful in organic chemistry, where carbon-based compounds form the heart of every study. Scientists use this kind of reaction to understand how to make new materials, like medicines, plastic parts, or even fuels. It’s also helpful in the classroom. Students get used to seeing chemical formulas and learning how components react. Every simple formula builds clear thinking skills, better science habits, and solid knowledge about how molecules work.
Where You Might See Similar Reactions in Real Life
You may be surprised to learn that the ideas behind hcooch ch2 h2o show up in everyday products. For example, when companies make plastic materials, tiny molecules like CH₂ units often get linked together to form long chains. In cosmetics or soaps, acids like formic acid sometimes help balance pH or preserve ingredients. And in nature, similar reactions happen inside your body! When your food breaks down and your cells create energy, chemical reactions involving small organic molecules often form water as a natural result. So, when learning about hcooch ch2 h2o, it’s not “just chemistry.” It’s a window into how things around you are made and how your own body works, hidden inside clever science.
The Role of Acids in Reactions Like hcooch ch2 h2o
Formic acid (HCOOH), seen in hcooch ch2 h2o, is an example of how acids help control reactions. Acids are molecules that donate protons (H⁺ ions) when they react. That might sound tricky, but here’s how to understand it simply. Think of acids as eager sharers—they give away a little part of themselves to get a reaction started. In hcooch ch2 h2o, formic acid might share part of its hydrogen to help bond with CH₂ or release a water molecule. Understanding acids teaches us why food spoils, how our stomachs digest, and even why batteries power our devices. Whether you’re dealing with a lemon or a lab test, acids are always active in chemistry—and this formula is a great way to learn how they behave.
How Learning hcooch ch2 h2o Helps Students
If you’re a teacher, parent, or student yourself, you might be thinking: “Why learn about hcooch ch2 h2o?” The answer is—it builds the foundation. This little formula helps you understand atomic bonding, acid reactions, and molecular groups. These lessons matter a lot when moving on to more complex ideas. It’s like learning how to walk before you run. Learning about this reaction improves your power to analyze, solve problems, and connect science to real life. Even kids can benefit. Use it for hands-on learning with molecule models, drawing atom diagrams, or creating fun classroom experiments. Keep it friendly, and learning chemistry becomes not just useful, but exciting too.
Teaching Tips: Explaining hcooch ch2 h2o to Young Learners
Want to make hcooch ch2 h2o fun for kids or beginners? Try using visual aids and real-life links. For CH₂, show a carbon-based Lego piece connecting to others. For HCOOH, talk about ants and their stings. And water, of course, is easy; it’s in every glass we drink. Use different colored balls or marshmallows to build molecule models. Try roleplay—one student can be “hydrogen,” another “carbon,” acting out how atoms join. You don’t need fancy lab tools to make chemistry meaningful. With simple creativity, even something like hcooch ch2 h2o turns into a lesson about teamwork among atoms!
FAQs
Q1: Is hcooch ch2 h2o a balanced equation?
Not fully. It likely represents a step within a larger process, rather than a complete chemical equation.
Q2: What is hcooch in the formula hcooch ch2 h2o?
“HCOOH” (mistyped here as hcooch) is formic acid, a simple and common organic acid.
Q3: What role does CH2 play in this reaction?
CH₂ is a reactive group that combines with other atoms, possibly helping form a product that releases water.
Q4: Does this reaction happen naturally?
Similar reactions happen in nature, like digestion, and in controlled lab environments using heat or catalysts.
Q5: Can beginners understand hcooch ch2 h2o in a science setting?
Yes! With basic tools, visuals, and good teaching, this simple equation helps explain bigger scientific ideas.
Q6: Why does this reaction matter in chemistry?
It introduces key ideas like acid behavior, molecular bonding, and water production, all of which are vital in organic chemistry.
Final Thoughts
Learning about hcooch ch2 h2o isn’t just about science class—it’s about understanding the little things that make the big stuff work. It connects small molecules like formic acid and tiny groups like CH2 into a simple story about chemical change. And with water as the end result, the story comes full circle in something that connects to all life. Whether you’re in school, teaching, or exploring chemistry for fun, this reaction is a great place to grow your knowledge. The next time you see a formula that looks tricky, remember: even the hardest things can be understood one atom at a time. Keep learning, keep experimenting, and keep asking questions—because science loves curious minds.